Recently, the emerging medical technology company Genesis MedTech and Shockwave Medical, a listed company in NASDAQ announced that the two parties will soon establish a joint venture company controlled by Genesis MedTech to introduce another world-leading new medical technology to China-Shockwave’s Intravascular lithotripsy (IVL). On the basis of the joint venture company, Genesis MedTech will establish a production line in China to convert and produce Shockwave’s products; at the same time, Genesis MedTech will also act as the exclusive agent of Shockwave in China to distribute the full range of the latter’s products in China.
Intravascular shock wave: a breakthrough innovation in calcification treatment technology
Plaque in blood vessels can cause blood vessels to narrow. Among patients with vascular stenosis, 30-50% suffer from calcified plaques. Calcified lesion is likely to occur in coronary arteries and peripheral arteries, which is extremely difficult to treat. At present, the interventional surgery for the treatment of calcified plaques is generally called “atherectomy/rotational atherectomy”, but this surgery is extremely difficult, and even for experienced surgeons, it is still a severe challenge. In addition, atherectomy/rotational atherectomy can only treat the superficial calcification of the vascular intima, and it is “helpless” for media calcification, calcified nodules or severe calcification. Moreover, the clinical prognosis of calcified diseases of the cardiovascular system is very poor, because calcified plaques are usually difficult to be expanded by ordinary balloons, and various complications are prone to occur even after expansion, which often leads to an increase in mortality.
 Shockwave products
Shockwave products
Shockwave Medical was established in California in 2009 and listed on NASDAQ in April 2019, focusing on the treatment of calcified lesions of the cardiovascular system. Shockwave applies extracorporeal shock wave lithotripsy for the treatment of kidney stones into blood vessels in an innovative manner. The acoustic pressure waves are delivered to the calcified part through a balloon catheter, and the calcified plaques are “vibrated” to restore the elasticity and blood flow of the blood vessels and remodel the diseased blood vessels; at the same time, it avoids the damage to the inner wall/intima of the blood vessel by the atherectomy/rotational atherectomy.
Shockwave’s products have been approved for clinical use in the European Union and the United States, and can be used to treat calcified lesions of coronary and peripheral arteries. Its products obtained the U.S. FDA breakthrough medical device certification in 2019, and its core technology has also obtained a number of invention patents in China. According to Shockwave, the product has been widely adopted across the world. Since its commercialization to October 2020, it has successfully treated more than 25,000 patients with coronary artery disease and more than 27,000 patients with peripheral artery disease.
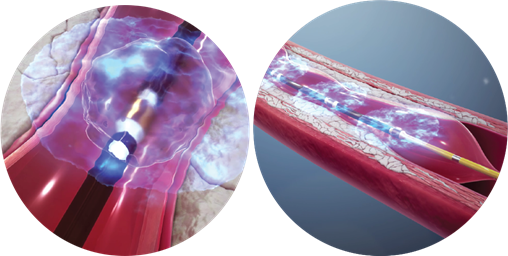
The picture shows how Shockwave’s products acts on blood vessels (Source: Shockwave’s official website)
Shockwave’s products and technologies have been highly evaluated in clinical practices abroad, and are generally recognized and accepted by domestic doctors. At the Zhejiang Heart Forum, 2020, Professor Chen Yundai of the Chinese PLA General Hospital mentioned that “calcified lesions have always been a huge challenge for interventionists. Nowadays, domestic surgeons mostly use non-compliant balloons, cutting balloons, excimer lasers, rotational atherectomy and other technologies to treat coronary artery calcification lesions, which proposes high requirements for clinical operations. And Shockwave’s intravascular shock wave calcification treatment technology is likely to be one of the new technological trends. ”
Simple to use, the innovative technology features a high success rate and a low risk of complications. It can safely and effectively treat calcified blood vessels, and is especially suitable for China where medical resources are unevenly distributed. As of now, there is no similar product on the market in China. Professor Fu Weiguo from the Department of Vascular Surgery of Zhongshan Hospital in Shanghai also spoke highly of the effect of Shockwave products in the treatment of peripheral diseases, hoping that this innovative product can be used by domestic patients as soon as possible.
Doug Godshall, President and CEO of Shockwave Medical, said: “We are excited to cooperate with Genesis MedTech. Genesis MedTech has established a strong R&D capability and marketing network in China, which can help our products accelerate into the Chinese market. We will work together with Genesis MedTech to bring innovative treatment methods to local patients. ”
Establish a Global Innovation Network: Genesis MedTech Builds Rich Product Lines for Peripheral and Cardiac Interventions
High-end medical equipment is an internationally recognized high-tech intensive industry. As an emerging domestic medical technology company, Genesis MedTech not only introduces and transforms new technologies, but also conducts independent research and development. At present, Genesis MedTech has R&D teams in Singapore, the United States, the Yangtze River Delta, Tianjin and other places, and conducts R&D cooperation with top teams in California, Israel and other countries to develop innovative products.
According to public information, in August 2020, Genesis MedTech acquired the “Chocolate® Touch Chocolate Balloons” assets of TriReme Medical LLC, and the Genesis MedTech team is responsible for the follow-up R&D, clinical trials, and product improvements of the product. Genesis MedTech’s Chocolate® Touch is used to treat peripheral arterial diseases, and the product combines the design of the Chocolate® Touch platform and the function of drug delivery at the same time. This breakthrough product can mitigate the damage to the vascular intima and reduce the use of salvage stents, providing safer and more effective treatment for patients with peripheral arterial diseases. Currently, the product is in the clinical registration stage and is expected to be launched in the United States in 2022.
With the acquisition of Chocolate® Touch technology and the establishment of a joint venture with Shockwave, Genesis MedTech already has two innovative products for the treatment of peripheral and coronary artery diseases. Capitalizing on the trend, Genesis MedTech has established a special business department within the group to be responsible for the R&D, clinical trials, registration, academic promotion and clinical services of peripheral and cardiac intervention products. According to industry insiders, Genesis MedTech has a number of products targeting peripheral diseases in the R&D. At the same time, Shockwave’s products will complete technology transfer and localization in the R&D and production base in Wuxi, Genesis MedTech.
“We hope to make use of our platform to incubate and accelerate domestic medical product innovation. Whether it is the independent R&D that we have been doing, or the introduction of Shockwave’s products and technologies, we hope that through our own R&D and transformation capabilities, we can help domestic patients obtain more advanced and high-quality medical resources” said Wang Xin, Chairman and CEO of Genesis MedTech.



